Dream Body Clinic Certified Stem Cells
Dream Body Clinic only provides certified human mesenchymal stem cells derived from Wharton Jelly (umbilical cord tissue) and placenta from live, healthy births. Our Lab is licensed by the Mexican health board COFEPRIS (Mexico’s FDA) to produce stem cells, to store stem cells as a cryo-bank and licensed to practice regenerative medicine. All 3 licenses are listed further down on this page. This guarantees that they have the highest quality standards in the collection, expansion and application of mesenchymal stem cells. This is to ensure a highly safe product that is free of pathogens. Our quality control is based on good manufacturing practices and the standards recommended by the International Society of Cell Therapy and the Latin American Mesenchymal Stem Cell Society of which we are members. We expand using a xeno-free culturing medium. The laboratory enters the qualification of ISO 6 (Cleanroom), which mean they meet the necessary requirements to make injectable products. We have specialized equipment such as Nuaire brand incubators and laminar flow hoods that guarantee the complete innocuousness of the cellular product. The cells are obtained from Wharton Jelly (Umbilical Tissue) and Placenta tissue which is extracted from previously selected donors under a rigorous medical screening that includes complete clinical history, serologic tests (HIV, Hepatitis C and B, syphilis, and Chagas), with high inclusion criteria so that they can be candidates for the donation. Once the sample has been obtained, several studies evaluated by third-party laboratories authorized by COFEPRIS are carried out, such as flow cytometry, microbiological tests (mycoplasma and endotoxins). Another step to our certified stem cells is that we do not take Wharton Jelly or Placenta from mothers that have had the Covid Vaccine. We feel that further study should be done on these before we take tissue from mothers that have had it due to the MRNA involved. We ask the mothers then verify with the federal database that lists all covid vaccine recipients in Mexico.





- Our stem Cells are never frozen. We take the stem cells out of cultivation the day before treatment and administer within 24 hours.
- We screen our umbilical cord donors. All donors must be between the ages of 18 and 25. They must be 1st time mothers and they must be married. We ask if they have received any COVID vaccines then we verify with their CURP number in the federal database.
- The first 3 passages of cultivation are to isolate pure mesenchymal stem cells as there are still other cell types at this point. We then cultivate to a maximum of 3 passages past this with the pure, isolated mesenchymal stem cells.
- All Patients receive a 3rd party analysis of their stem cells just like in the example below.
Example of Stem Cell Analysis Below:
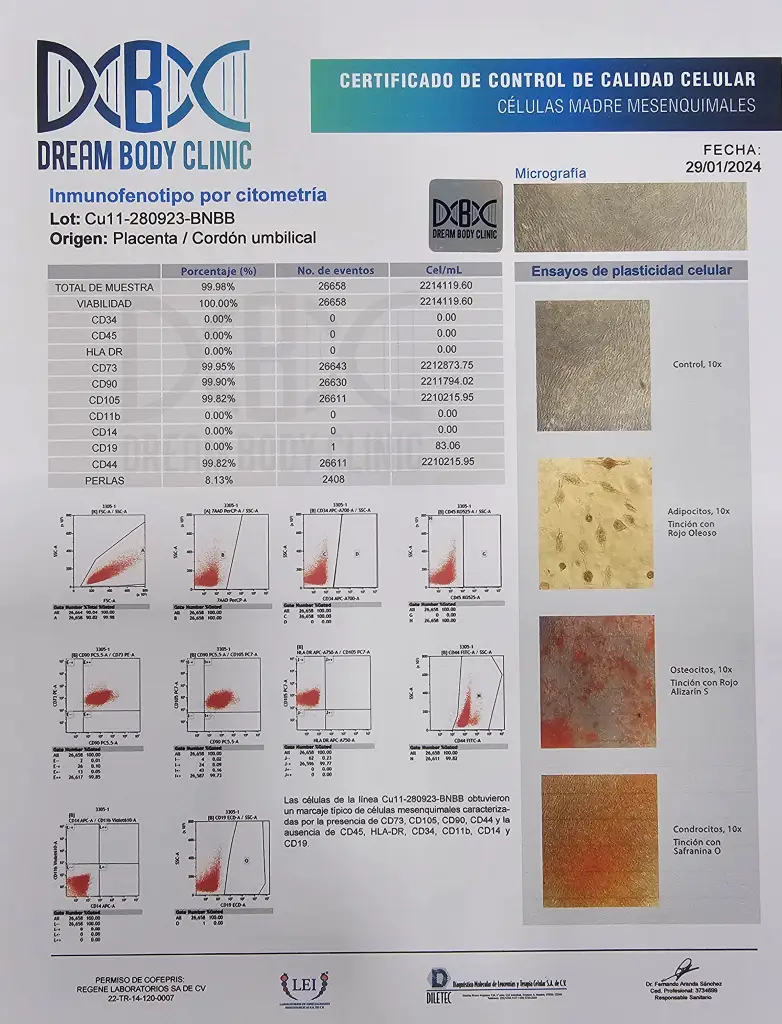
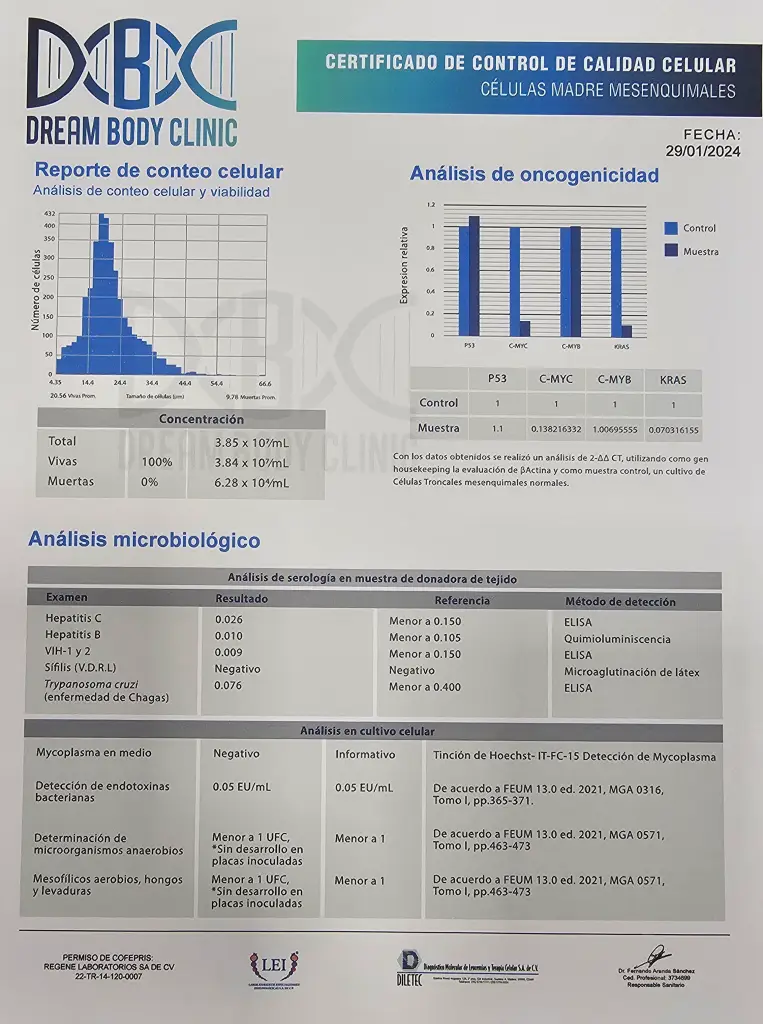
Below are our Licenses and Documentation for our Clinic and Lab


