Effects of Progesterone on Sleep: A Possible Pharmacological Treatment for Sleep-Breathing Disorders? Study Review
Effects of Progesterone on Sleep: A Possible Pharmacological Treatment for Sleep-Breathing Disorders? Study Review by Dream Body Clinic:
The study, “Effects of Progesterone on Sleep: A Possible Pharmacological Treatment for Sleep-Breathing Disorders?” by M.L. Andersen et al., explores the multifaceted role of progesterone beyond its traditional association with female reproduction. Progesterone influences various physiological and psychological functions, including sleep quality, respiration, mood, and cognitive processes. This review highlights progesterone’s potential therapeutic benefits in treating sleep-disordered breathing, such as sleep apnea, particularly noting its hypnotic and respiratory stimulant properties.
Key points from the study include:
Progesterone’s Broad Biological Impact: Beyond its well-known role in female reproduction, progesterone affects a wide range of biological activities, including sleep and respiration. It acts as a sleep inducer and a potent respiratory stimulant, potentially decreasing episodes of sleep apnea in men and addressing obesity-hypoventilation in menopausal women.
Mechanisms of Action: Progesterone’s effects on sleep and breathing are mediated through its interaction with various receptors, including those in the brain responsible for regulating sleep architecture and respiratory function. Its conversion into neurosteroids like allopregnanolone, which modulate GABA_A receptors, contributes to its anxiolytic and sedative effects.
Therapeutic Potential: The review suggests progesterone’s utility in treating sleep-disordered breathing, highlighting its ability to stimulate respiration and improve sleep quality. This is particularly relevant for conditions like obstructive sleep apnea (OSA), where progesterone could offer a pharmacological alternative to mechanical treatments like CPAP (Continuous Positive Airway Pressure) machines.
Gender Differences and Hormonal Influence: The study discusses how progesterone levels and their effects on sleep and breathing differ between genders and across different life stages, such as menopause. This underscores the hormone’s potential role in the gender disparity observed in sleep-disordered breathing conditions.
Research Gaps and Future Directions: Despite promising findings, the review acknowledges the need for more research to fully understand progesterone’s impact on sleep and breathing disorders, especially in males. It calls for further studies to explore long-term effects and the potential for progesterone-based therapies.
In conclusion, Andersen et al.’s review positions progesterone as a hormone of significant interest beyond its reproductive functions, with potential applications in treating sleep and respiratory disorders. The study underscores the importance of hormonal regulation in sleep and breathing, suggesting new avenues for therapeutic interventions.
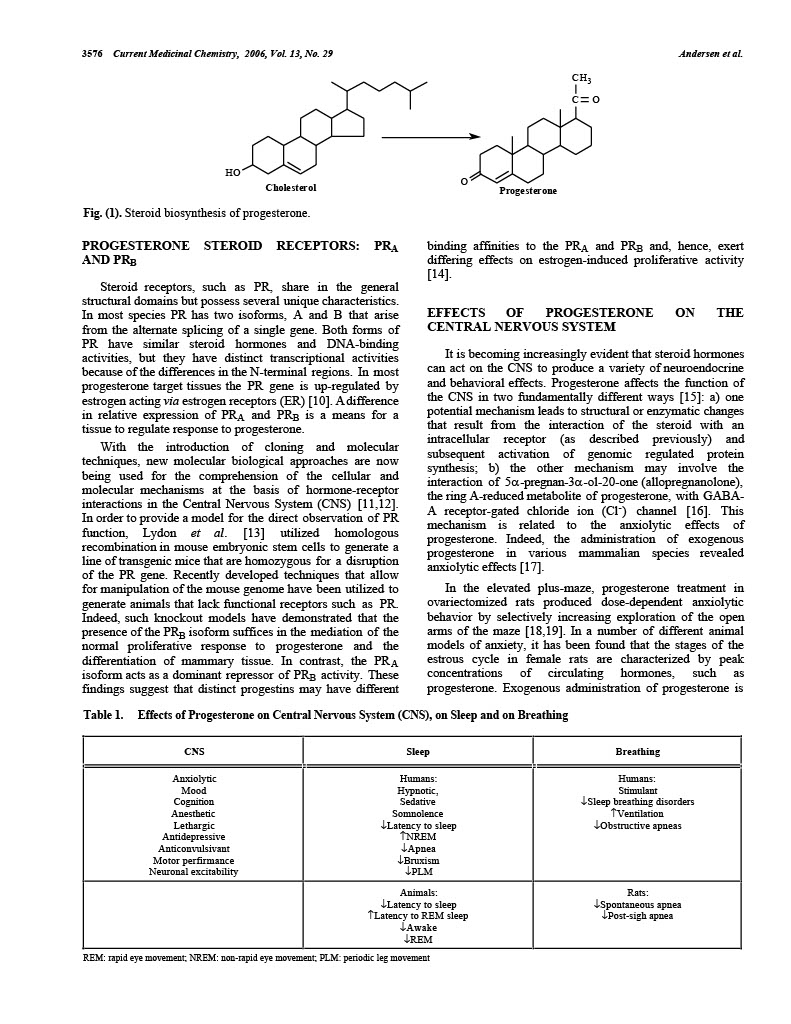
Effects of Progesterone on Sleep: A Possible Pharmacological Treatment for Sleep-Breathing Disorders? M.L. Andersen*, L.R.A. Bittencourt, I.B. Antunes and S. Tufik Department of Psychobiology – Universidade Federal de São Paulo, Escola Paulista de Medicina (UNIFESP/EPM), R. Napoleão de Barros, 925, V. Clementino 04024-002, São Paulo, SP, Brazil Abstract: Progesterone is present in a wide spectrum of biological activity within a variety of tissues. This hormone is also known to affect reproduction, sleep quality, respiration, mood, appetite, learning, memory and sexual activity. Progesterone exerts a sleep induction or hypnotic effect and is a potent respiratory stimulant that has been associated to a decrease in the number of central and obstructive sleep apnea episodes in men. The literature also contains a substantial amount of data on the effect of apnea in women with obesityhypoventilation during menopause. This review attempts to outline the specific role of progesterone in normal sleep and breathing as well as its possible therapeutic effects in the treatment of sleep-disordered breathing. Keywords: Progesterone, sleep, sleep apnea, breathing, hormonal control. INTRODUCTION The association between steroid hormones and brain function has traditionally been perceived within the confines of, and limited to the realm of, endocrine mechanisms. Such view is justified since endocrine mechanisms are propelled into action as responses elicited by secretory products of steroidogenic endocrine glands, carried by the bloodstream, and exerting actions on the brain, as this organ indeed is a target for steroid hormones. Intracellular receptors involved in the regulation of specific gene transcription have been identified in neuroendocrine structures. Every class of receptors has a distinct distribution pattern in the complex anatomy of the brain [1,2]. Mechanisms involving nuclear receptors account for most steroid-induced feedback as well as for many behavioral effects in the regulation of the synthesis of several neurotransmitters, hormonemetabolizing enzymes, hormone and neuromediator receptors, and for the organizational effects on neural circuitry [3]. Hormones such as progesterone exert a wide spectrum of biological activity in a variety of tissues. The effects of progesterone can be stimulatory or inhibitory depending upon the tissue involved, the dose of progesterone used, and its time of administration [4]. Progesterone is well known for its central role in female reproduction and a great deal of research has focused on elucidating the neural and endocrine mechanisms of progesterone in females. Although sleep has been a subject of endocrinological research, less attention however, has been directed to the possible role of progesterone on sleep architecture and in the sleep electroencephalogram (EEG) in both genders, and to its influence in respiration especially in males in which sleepdisordered breathing is more prevalent. *Address correspondence to this author at the Department of Psychobiology – Universidade Federal de São Paulo, Rua Napoleão de Barros, 925, Vila Clementino – SP- 04024-002, São Paulo, Brazil; Tel: (5511) 2149-0155; Fax: (55-11) 5572-5092; E-mail: mandersen@psicobio.epm.br 0929-8673/06 $50.00+.00 PROGESTERONE: A STEROID HORMONE Although progesterone is a sex steroid, it stands at an important crossroad of these pathways and may be converted either into other sex steroids, into glucocorticoids, or into 3α,5α-reduced neurosteroids [5]. Thus, progesterone is of paramount importance and essential in the synthesis of other hormones, specifically of supra-renal corticosteroids, estrogen and testosterone. The term “progestogens” encompasses the endogenous hormone progesterone and the synthetic steroids that mimic progesterone – progestins. The pharmacological properties of progestogens (by definition progestins) vary depending upon the molecules from which they are derived (either progesterone or testosterone), and differences can lead to a broad range of activity of the derivate [6]. The effects of progestins are related to interactions not only with the progesterone receptor (PR), but also with other steroid receptors, such as androgen, estrogen, glucocorticoids and mineralocorticoid receptors. Such interactions may either induce transactivation or prevent activation of the steroid receptor, and may be responsible for some of the undesirable side effects of progestins [7]. Pregnenolone is the precursor of progesterone and is catalyzed by 3β-hydroxysteroid dehydrogenase. The main serum metabolites of progesterone are 17α-OH-progesterone (catalysed by 17α-hydroxylase), deoxycorticosterone (catalysed by 21-hydroxylase), as well as the main urinary metabolite pregnanediol. 17α-OH-progesterone leads to 11deoxycortisol, which in turn produces cortisol as well as androstenedione and dehydroepiandrosterone. These are intermediate steroids in the biosynthesis of androgens and estrogens (for complete review, [8]). Progesterone is synthesized in the adrenals, ovaries and also in the Leydig cells of testicles and exhibits both anti-androgenic and antimineralocorticoid effects. Through competitive inhibition, progesterone prevents the conversion of testosterone into its active metabolite, dihydrotestosterone, by the enzyme 5αreductase. Progesterone also binds competitively to the mineralocorticoid receptor, preventing its transactivation and inhibiting the mineralocorticoid effect [9]. © 2006 Bentham Science Publishers Ltd.3576 Current Medicinal Chemistry, 2006, Vol. 13, No. 29 C C O Andersen et al. H3 HO Cholesterol Fig. (1). Steroid biosynthesis of progesterone. PROGESTERONE STEROID RECEPTORS: PRA AND PRB Steroid receptors, such as PR, share in the general structural domains but possess several unique characteristics. In most species PR has two isoforms, A and B that arise from the alternate splicing of a single gene. Both forms of PR have similar steroid hormones and DNA-binding activities, but they have distinct transcriptional activities because of the differences in the N-terminal regions. In most progesterone target tissues the PR gene is up-regulated by estrogen acting via estrogen receptors (ER) [10]. A difference in relative expression of PRA and PRB is a means for a tissue to regulate response to progesterone. With the introduction of cloning and molecular techniques, new molecular biological approaches are now being used for the comprehension of the cellular and molecular mechanisms at the basis of hormone-receptor interactions in the Central Nervous System (CNS) [11,12]. In order to provide a model for the direct observation of PR function, Lydon et al. [13] utilized homologous recombination in mouse embryonic stem cells to generate a line of transgenic mice that are homozygous for a disruption of the PR gene. Recently developed techniques that allow for manipulation of the mouse genome have been utilized to generate animals that lack functional receptors such as PR. Indeed, such knockout models have demonstrated that the presence of the PRB isoform suffices in the mediation of the normal proliferative response to progesterone and the differentiation of mammary tissue. In contrast, the PRA isoform acts as a dominant repressor of PRB activity. These findings suggest that distinct progestins may have different O Progesterone binding affinities to the PRA and PRB and, hence, exert differing effects on estrogen-induced proliferative activity [14]. EFFECTS OF PROGESTERONE ON THE CENTRAL NERVOUS SYSTEM It is becoming increasingly evident that steroid hormones can act on the CNS to produce a variety of neuroendocrine and behavioral effects. Progesterone affects the function of the CNS in two fundamentally different ways [15]: a) one potential mechanism leads to structural or enzymatic changes that result from the interaction of the steroid with an intracellular receptor (as described previously) and subsequent activation of genomic regulated protein synthesis; b) the other mechanism may involve the interaction of 5α-pregnan-3α-ol-20-one (allopregnanolone), the ring A-reduced metabolite of progesterone, with GABAA receptor-gated chloride ion (Cl-) channel [16]. This mechanism is related to the anxiolytic effects of progesterone. Indeed, the administration of exogenous progesterone in various mammalian species revealed anxiolytic effects [17]. In the elevated plus-maze, progesterone treatment in ovariectomized rats produced dose-dependent anxiolytic behavior by selectively increasing exploration of the open arms of the maze [18,19]. In a number of different animal models of anxiety, it has been found that the stages of the estrous cycle in female rats are characterized by peak concentrations of circulating hormones, such as progesterone. Exogenous administration of progesterone is Table 1. Effects of Progesterone on Central Nervous System (CNS), on Sleep and on Breathing CNS Sleep Anxiolytic Mood Cognition Anesthetic Lethargic Antidepressive Anticonvulsivant Motor perfirmance Neuronal excitability Breathing Humans: Hypnotic, Sedative Somnolence ↓Latency to sleep ↑NREM ↓Apnea ↓Bruxism ↓PLM Humans: Stimulant ↓Sleep breathing disorders ↑Ventilation ↓Obstructive apneas Animals: ↓Latency to sleep ↑Latency to REM sleep ↓Awake ↓REM Rats: ↓Spontaneous apnea ↓Post-sigh apnea REM: rapid eye movement; NREM: non-rapid eye movement; PLM: periodic leg movementProgesterone and Sleep Current Medicinal Chemistry, 2006 Vol. 13, No. 29 3577 associated with behavior that is commonly observed following the acute administration of benzodiazepines (BDZ, [20]). The mechanism relating the anxiolytic effects to progesterone is based on the following: a) the prototypical anxiolytic BDZ drugs act by binding to a site associated with the GABAA receptor; b) like BDZ, allopregnanolone increases the binding affinity of 3H-muscimol, a GABA A receptor agonist; c) similar to barbiturates, the in vitro addition of allopregnanolone increases the duration of Clchannel opening elicited by GABA; d) progesterone and allopregnanolone injection produces anxiolytic, anticonvulsant, analgesic, anti-aggressive and sedative-hypnoticanesthetic effects (for review see Bitran et al. [19]), most of which are commonly observed after BDZ administration [21]. Taken together, these consistent experimental data indicate that the behavioral effects observed after administration are due to its biotransformation to pregnenolone and allopregnanolone, which subsequently augment GABAA receptor-mediated functions. EFFECTS OF PROGESTERONE ON SLEEP a). Physiology of Sleep Sleep is a cyclical or episodic phase that alternates with wakefulness. An important characteristic of sleep that differentiates it from most other states of altered consciousness is that it is promptly reversible. Electrophysiological studies in the 1950s demonstrated that there were two main states of sleep, non-rapid eye movement (NREM) and rapid eye movement (REM) sleep [22,23]. The lighter stages of NREM sleep (phase 1 and 2) appear first, and often alternate with brief episodes of wakefulness before deeper NREM sleep stages set in. NREM sleep, and particularly its deeper stages (phase 3 and 4) predominate early in the night, but REM sleep appears at around 90 minute intervals. There are usually 4-6 of such sleep cycles each night and as night progresses REM episodes become longer, and NREM sleep both shorter and lighter. The NREM sleep is recognized by low frequency and high amplitude, the presence of sleep spindles and hypotonia, registered on an EMG. REM sleep probably enables behavior patterns and emotional responses to become established. During REM sleep information obtained during wakefulness appears to be reprocessed and integrated into existing neural templates so that future responses can be modified or matured to reflect both the experience of the individual and the inherited potential [24]. The electrophysiological features of REM sleep are the combination of a wide range of desynchronized EEG frequencies, loss of EMG activity and the presence of rapid eye movement. The EEG reflects the intense cerebral cortical activity that distinguishes REM from NREM sleep, but its similarity to the EEG of wakefulness also led it to be called “paradoxical” sleep (PS). b). Variations in Progesterone Concentrations Among the mechanisms of neuroendocrine control, the circadian rhythm is superimposed on episodic secretion. It is the result of CNS events that regulate both the number and magnitude of secretory episodes of corticotropin-releasing hormone (CRH). In particular, data are contradictory concerning age-dependent alterations in blood concentrations of progesterone. No significant diurnal changes in salivary progesterone concentrations were found in healthy newborns [25], however progesterone was highest in this age group compared with the other age groups. At 1-12 months, children have an one-third decrease in relation to newborn concentrations. According to the authors, the circadian rhythm was fully developed in children over two years of age. In pubertal adolescents, the values were distributed over a broader range. In adult men (age 40 to >60), progesterone concentrations showed a significant decrease in function of age [26]. Fluctuations in secretion patterns of progesterone may be accompanied by mood disturbances, impairment of physical well-being, vigilance state, and sleep quality. Although it has been demonstrated that sex hormones influence sleep and that sleep has been shown to induce changes in pituitary hormone secretion [27], several questions remain unanswered. Reference ranges for blood production rates (PR), metabolic clearance rates (MCR) and secretion rates (SR) for progesterone in normal menstruating women in follicular phase is 2.0 mg/day, 1.7 mg/day and 2100 L/day, respectively. In the luteal phase of menstrual the cycle, values are 25 mg/day, 24 mg/day and 2100 L/day [28]. A discrepancy was found in the Belanger study. Serum concentrations steroids were measured in 2423 men aged 40 to 80 years. The small decrease in progesterone led to no age-related alterations [29]. Recently, Oettel and Mukhopadhyay (2004) [8] reviewed the theme and likewise stated that there were no age-dependent alterations in serum progesterone in neither men nor postmenopausal women. c). Effects of Progesterone on Sleep Pattern Progesterone also rapidly decreases neuronal excitability, as shown in vivo by its hypnotic anaesthetic properties [30]. Based on Selye reports [30] the anaesthetic effects of large doses of steroids administered to animals by intravenous and intraperitoneal routes are well documented, but in humans the data available are not so dissected. A dose-dependent hypnotic effect after progesterone was reported in the mid 1950s [31] and the consistency with which the intravenous administration of progesterone has been associated with somnolence [31] have prompted some studies to focus on this theme. The precise effects of progesterone on sleep architecture and the sleep electroencephalogram (EEG) are yet to be well documented. Studies on the influence of exogenous progesterone on vigilance focused mainly on its anesthetic potency as measured by parameters such as loss of righting reflex and increase in cortical arousal threshold [32,33]. In women, sleep was investigated during phases associated with physiological changes in progesterone concentrations, over the menstrual cycle and during the menopausal period. These results do not determine whether such alterations are due to aging, which by itself would alter sleep quality, or due to the menopause status. In any case, there is a lower sleep efficiency [34], greater sleep latency [35] and difficulty3578 Current Medicinal Chemistry, 2006, Vol. 13, No. 29 Andersen et al. to maintain sleep [36,37] in post-menopausal than in premenopausal women. Normally, hormone replacement therapy with medroxyprogesterone improved sleep quality, reducing complaints of snoring, bruxism and apnea, as well as periodic leg movements [38]. Initial reports of the effects of progestins on the central neuronal excitability of healthy male subjects measured by a quantitative spectral analysis of the EEG during wakefulness have indicated certain similarities between the patterns of EEG power spectra induced by progestins and benzodiazepines [39,40]. For instance, intravenous injections (100-500 mg) produced hypnotic effects in which the patients fell asleep during the first minutes during the injection and some effort was needed to arouse the subjects. Later it was described that the dose of 400 mg of micronized progesterone orally induced a progressive lethargy and drowsiness in a 55-year old woman. Approximately one hour after receiving this hormone, this subject fell into deep sleep that lasted about an hour [41]. A detailed sleep-endocrine study in humans using a double-blind, placebo-controlled crossover design evaluated the effects of orally administered progesterone on sleep architecture and sleep-associated hormone secretion in healthy male volunteers [42]. The dose of 300 mg micronized progesterone induced a significant increase in the amount of non-rapid eye movement (NREM) sleep. The EEG spectral power during NREM showed a significant decrease in the slow wave frequency range, whereas the spectral power in the higher frequency range tended to be elevated. No significant effects of progesterone administration on REM sleep variables were detected. Some of the observed changes in sleep architecture and sleep-EEG power spectra are similar to those obtained with agonistic modulators of the GABAA receptor by a decrease within the delta frequency range and an increase in the beta frequency range [42]. Very few studies have focused on whether progesterone dosages that influence sleep produce sedative hangover effects. Thus, Gron and co-workers [17] assessed the effects of a single oral dose of 300 mg micronized progesterone administration in healthy male volunteers. The administration of progesterone produced no consistent effects on attention performance, leading the authors to affirm that the dosages of progesterone that modulate sleep are not likely to exert sedative after-effects. In animal models, the influence of progesterone has also been investigated. Lancel et al. [43] evaluated the effects of three nonanesthetic doses of progesterone on the amount of time spent in the different vigilance states and on sleep statespecific EEG activity in male rats. Progesterone administration resulted in dose-dependent shortened NREM sleep latency, lengthened REM sleep latency, and decreased awake time and REM sleep. In NREM, EEG activity was reduced in the lower frequencies and was enhanced in the higher frequencies. The effects of progesterone on sleep pattern closely resemble those induced by GABAA agonists such as BDZ in rats. Later, the same group assessed the involvement of GABAA receptors by investigating the sleep responses to GABAA antagonist. The previous findings with progesterone on sleep were effectively attenuated by the GABAA picrotoxin that delayed the latency to NREM sleep and thereby decreased all sleep states. Taken together, the progesterone-induced sleep alterations seem to be due to a potentiation of GABAA receptor-mediated responses evoked by its neuroactive metabolites(s) [44]. Particular attention has to be drawn to neuropsychopharmacological properties of 3α-reduced neuroactive steroids. Since progesterone and 3-α reduced neurosteroids are known to bind to progesterone receptors (for review see Rupprecht [45]), the use of antiprogestins (such as mifepristone) provide the unique opportunity to study the effects of reversible progesterone blockade on a variety of physiological and pharmacological processes [46]. Further, it could be stated that mifepristone should inhibit sleep effects, possibly in response to nuclear receptors. Although we were not able to observe any significant alteration in sleep efficiency, REM sleep was reduced after mifepristone in male rats [47]. Taken together, these facts are consistent with the hypothesis that progesterone must be involved in sleep regulation and might play a functional role in the regulation of different behavior in males (such as sexual behavior – for review see Andersen and Tufik [48]). The complete comprehension of the mechanisms involved in its effects remains open to speculation. EFFECTS OF PROGESTERONE IN BREATHING a). Respiration during Sleep During sleep factors such as loss of voluntary or cortical control of ventilation, decrease of ventilatory response to hypoxemia and hypercapnia, as well as reduced tonus of the upper airway and thoracic muscles lead to distinct patterns of respiration in the different stages of sleep. In unsteady NREM sleep (stages 1 and 2) there occurs a pattern of periodic breathing with periods of hyperventilation and alternating episodes of hypoventilation and central apnea. As sleep consolidates the number of arousals diminishes and breathing becomes more regular and with a higher intensity of hypoventilation than that of the preceding phase. This period is considered stable NREM sleep (stages 3 and 4). When REM sleep sets in subsequently, and the respiratory patterns become irregular, variability in breathing frequency is observed with possible resumption of central apnea [49]. Respiration as a vital function is not regulated by specific hormones, but rather, is influenced by a wide array of hormones [50]. The current bulk of evidence suggests that hormones have a relevant role in the regulation of breathing via several mechanisms. They may stimulate breathing at the level of the CNS or at peripheral chemoreceptors (eg. progesterone) or by altering the basic metabolic rate, also indirectly affecting breathing (eg. thyroid hormones). The effects of progestational agents on chemosensitivity were initially described by Lyons and Antonio [51] who found that hypercapnic ventilatory response increased when progesterone was administered to three normal men. Later, Zwillich et al. [52] reported that medroxyprogesterone acetate daily in 11 normal men increases resting minute ventilation and chemosensitivity as measured by the ventilatory response to hypercapnia and hypoxia. The ventilatory stimulant effect of medroxyprogesterone acetate have been suggested to be due to central mechanims [53].Progesterone and Sleep Current Medicinal Chemistry, 2006 Vol. 13, No. 29 3579 Progesterone is a potent respiratory stimulant, whereas the role of the other reproductive hormones in the control of breathing has not been established [50]. The multiple location of progesterone, estrogen, androgen, prolactin, and human chorionic gonadotropin/luteinizing hormone receptors suggests that these hormones might act locally in various tissues, including trachea, lungs, brain, and brainstem [5456], and thereby possibly be involved also in breathing. The beneficial effects of progesterone on the upper airway function and breathing are also supported by animal studies. In decerebrated cats, pretreatment with progestins decreases the alcohol-induced mismatching of hypoglossal and phrenic activities [57]. b). Sleep-Related Breathing Disorders The marked sleep (among other) alterations during the menstrual cycle and menopausal period have led to the execution of several studies. Menopause has a major impact on sleep and breathing, and sleep disorders are less common in women until after menopause [58] but still remain less frequent than in men. The main breathing disorders associated to sleep syndromes are: obstructive sleep apnea (OSA), central sleep apnea and hypoventilation [59]. In fact, OSA is an extremely common condition that compromises the vital functions of respiration and circulation. Very few studies have examined the influence of sex on upper airway resistance during sleep. In this sense, Driver and colleagues [60] demonstrated a significant reduction in upper airway resistance during sleep in the luteal phase of the menstrual cycle when compared with the follicular phase in 11 healthy women. The authors suggest that the mechanism for this result could be attribute to the stimulant effect of progesterone on the upper airway musculature. Thus, the peak of progesterone in the luteal phase may play a significant role in protecting premenopausal women from sleep-disordered breathing [60]. The reasons for the gender difference, and the increased prevalence of OSA in post-menopausal when compared to their pre-menopausal counterparts are still not completely comprehended, but estimates of the prevalence of this condition among women in their sixth or seventh decade of life range from 4% to as high as 22% [61,62], depending on the definition used and the population that was examined. It has been documented that, along with other biologic consequences of aging, the transition to the postmenopausal period and the associated hormonal changes might increase the risk of developing OSA [63,64] or might yet exacerbate a pre-existing disorder [65]. Considering female sex hormones may protect against the development of OSA, androgens may promote the disorders (see Cistulli et al. and references therein [66]). Differences in upper airway structure/function and/or ventilatory control during sleep could support the gender difference, although the relative contribution of each mechanism is uncertain [67]. A large upper airway soft tissue volume in men may contribute to the increased prevalence of obstructive sleep apnea in men compared to woman [68] and upper airway resistance is higher in men than it is in women [69]. However, no gender comparison has been done during sleep. Furthermore, the difference in upper airway structure does not account for the mentioned difference in the prevalence of sleep apnea [67]. Among the sleep-disordered breathing, OSA is a highly prevalent syndrome that is increasingly recognized as the cause of a variety of clinical problems. Heavy snoring, excessive daytime sleepiness, hypertension, sexual dysfuntion, etc., can all be manifestations of this relatively common and potentially life-threatening disorder. The increased prevalence of sleep apnea in postmenopausal women may be related to the decline in estrogen and progesterone concentrations after the menopause. c). Use of Progesterone in the Treatment of SleepDisordered Breathing Synthetic progestins have been used for respiratory stimulation with variable success [70,71]. As stated previously, few studies have reported some improvements in sleepiness, blood gas levels, and in the number or duration of apneic and hypopneic events [72]. Very few such studies were conducted on males. Progestins and estrogen have been shown to reduce mild OSA in post-menopausal women [38,73]. In addition, progestational hormones have been demonstrated to stimulate ventilation during the luteal phase [74] as well as medroxyprogesterone acetate at a daily dose of 60 mg/day for two weeks improves ventilation in post-menopausal women with partial upper airway obstruction during sleep without compromising sleep [75]. Thus, the higher prevalence of sleep apnea in post-menopausal subjects than that observed in pre-menopausal women suggest that the female hormones, particularly progesterone, may protect premenopausal women from developing obstructive sleep apnea syndrome (Kapsimalis and Kryger [63,64]). It had been proposed that female sex hormones could potentially protect women from OSA by slowing the rate of ventilatory decline after a brief stimulus. This could occur through either a general upregulation of the respiratory controller or a direct increase in neuronal short-term potentiation [76]. Thus, there is potential for female sex hormones to modulate the phenomenon of neural short-term potentiation [76]. In addition to obesity and structural abnormalities in the upper airway, a decrease in the drive to breathe is likely to be involved in hypoventilation in postmenopausal obese females. Thus, decreased production of progestins after menopause may result in lack of natural respiratory stimulation that predisposes women to respiratory insufficiency. Therefore, progestin replacement therapy could effectively improve ventilation and oxygenation during sleep [75]. The authors demonstrated that a trial with 60mg/day/14 days of medroxyprogesterone acetate in postmenopausal women with moderate to severe chronic pulmonary disease (COPD) induced consistent and prolonged respiratory improvement. A previous investigation found that that 17% of healthy postmenopausal females spent a significant portion of their sleep time with partial upper airway obstruction [77]. Since partial upper airway obstruction predisposes afflicted individuals to carbon dioxide (CO2) retention, and to the respiratory stimulating effects of medroxyprogesterone acetate, the authors reported that progesterone improves ventilation in3580 Current Medicinal Chemistry, 2006, Vol. 13, No. 29 Andersen et al. the affected females. Such betterment was maintained beyond 3 weeks post-treatment. Nasal continuous positive airway pressure (nCPAP) was more efficient than medroxyprogesterone in decreasing respiratory efforts but the end-tidal pressure of carbon dioxide decreased that effort more so with hormone therapy [75]. Based on the fact that medroxyprogesterone acetate acts as a respiratory stimulant, it might increase respiratory drive to upper airway muscles as well as to the diaphragm and intercostal muscles, Strohl and colleagues [78] hypothesized that this hormone treatment could diminish obstructive apneas during sleep and relieve its symptoms. The results demonstrated that besides symptomatic improvement, medroxyprogesterone responders significantly decreased the number of obstructive apneas during sleep when compared with non-responders. The apneic males that responded to the treatment had lower PaO2 values compared to non-responders and further, tend to be more hypercapnic [78]. In this regard, and in addition to sleep apnea, the male responders might also have obesity-related hypoventilation. The progesterone treatmentinduced respiratory stimulation could supposedly work better in those male patients whose OSA overlaps with respiratory insufficiency [50]. Strohl et al. [78] proposed that relief of obstructive apneas played a major role in the clinical improvement verified in a previous study [79] with medroxyprogesterone therapy in obesity-hypoventilation syndrome, and suggested that their data could stimulate additional investigation of medical treatment for obstructive apneas during sleep. Later, Rajagopal et al. [80] studied the effects of medroxyprogesterone on the incidence and duration of episodes of apnea in 13 non-hypercapnic men with obstructive sleep apnea. The authors conducted a prospective study with polysomnography recording that was done before and after four weeks of treatment with medroxyprogesterone acetate (60 mg/day), and one week after cessation of the treatment. The result demonstrated that in apneic patients who are non-hypercapnic during wakefulness, progesterone improved respiratory drive response to hypercapnia, but did not improved the respiratory events during sleep. Orr and his colleagues [81] reported failure of medroxyprogesterone to alter obstructive sleep apnea in seven patients, but a symptomatic improvement was mentioned. The literature on this theme has produced conflicting results [82]. This scenario prompted a randomised doubleblind crossover study designed by Cook et al. [83]. Placebo or 150 mg/day of medroxyprogesterone acetate were administered to males with obstructive sleep apnea during a week. The treatment did not induce changes in sleep parameters in any of the hypercapnic or normocapnic patients. Using chlormadinone acetate, a respiratory stimulant known to increase not only CO2 and hypoxic chemosensitivity but also respiratory drive response for ventilatory loading, Kimura et al. [84] tested its possible beneficial effect in nine sleep apneic patients. The data indicate that chlormadinone acetate was effective in patients suffering of sleep apnea syndrome (reduced apnea and hypoxic episodes) whose load response as well as respiratory control activity are augmented during wakefulness. Although there have been many studies on the effects of progesterone, the relationship between the effectiveness on sleep-disordered breathing and the influence of progesterone on control of breathing during wakefulness still remains obscure. It has been proposed that relative duration of the desaturation period in total sleep time is a relevant determinant. Such evaluation might have resolved, at least in part, the apparent discrepancies in previous studies [84]. In general, patients with obstructive sleep apnea are most effectively treated with the promising continuous positive airway pressure (CPAP) and/or surgery. For patients who are not surgical candidates or refuse to use CPAP, drug therapy may be beneficial. In 1997, Terra and Oberg [85] suggested that medroxyprogesterone acetate should be reserved for hypercapnic patients who refuse other modalities of treatment. The current knowledge on the efficacy of progestins to control obstructive sleep apnea is based on studies in males. As pointed by Saaresranta et al. [75], few studies have focus on postmenopausal females with sleep-disordered breathing using medroxyprogesterone acetate in combination with estrogen. Their common conclusion was that there was some decrease in apnea hipoapnea index (AHI) or shortening of apnea duration. In postmenopausal females with chronic respiratory failure, the ventilatory improvement was sustained for at least three weeks after cessation of the hormone [86]. As stated by the authors, the sustained ventilatory effect of short-term medroxyprogesterone acetate suggest that periodic administration might be sufficient to improve ventilation in females. In terms of sleep efficiency, periodic administration might even be superior to continuous therapy [75]. Animal models have also been conducted to investigate the role of progesterone in sleep-disordered breathing. Recently, Yamazaki and colleagues [87] examined the effects of two doses of progesterone on the apneic events during behaviorally defined sleep in male rats at 4, 14 and 26 weeks of age. Their finding indicated that the number of events of spontaneous apnea (SA) and post-sigh apnea (PSA) increased with age and the duration of apneic events were prolonged in older rats. In the group treated with 1 mg/kg of progesterone, the number of both significantly decreased SA and PSA, and inhibition was increased in an age-dependent manner. The dose of 30 mg/kg, had no effect on the number of SA and PSA, although it prolonged the duration of PSA. The pretreatment with mifepristone (antagonist of PR), inhibited the effects of the lowest dose of progesterone, but did not result in any antagonistic effect on the high doseinduced changes. As suggested by the authors, the progesterone-mediated mechanisms are involved, at least in part, in respiratory function during sleep in rats, and thereby supports the therapeutic usage of progesterone at proper doses. The inhibition of progesterone effect by mifepristone in the number of sleep apneic events supports this hypothesis and it would not be unreasonable to extrapolate that such treatment would benefit humans, reducing hypoventilation and central apnea. CONCLUSIONS Named for its central role in female reproduction, progesterone traditionally has been thought to have little orProgesterone and Sleep [15] Current Medicinal Chemistry, 2006 Vol. 13, No. 29 3581 no relevance in males. The review of progesterone on sleep as well as its disturbances in male subjects rule out interference from variations in endogenous progesterone secretion found in females. Although there has been increased interest in all aspects of sleep function/disorders, and the importance of hormones in the regulation of sleep has long been well documented, knowledge of the role of progesterone on male sleep architecture and sleep-disordered breathing is still uncertain. Few human studies exist on this theme, and most of the literature concerns female research, in particular during preor post-menopausal periods. Although little information on the effect of progesterone on sleep disturbances in males is currently available, recent studies have provided valuable insight of progesterone modulating apneic events in male rats [87]. Such data show that this hormone plays a more relevant role in the control of sleep than commonly assumed and reinforces the importance of the interaction of hormones and breathing, in addition to offering new perspectives for novel pharmacologic therapies of sleep breathing disorders. Certainly more studies are warranted and long-term adverse effects of progesterone compounds must be addressed to elucidate the beneficial effects of progesterone on sleeprelated breathing disorders in males.