Progesterone-induced changes in sleep in male subjects Study
This is a review of : Progesterone-induced changes in sleep in male subjects study by dream body clinic.
The study titled “Progesterone-induced changes in sleep in male subjects” by Elisabeth Friess et al. investigates the effects of progesterone administration on sleep patterns, specifically focusing on the sleep electroencephalogram (EEG) in healthy male subjects. This double-blind, placebo-controlled crossover study involved nine healthy male volunteers and aimed to understand how progesterone affects sleep architecture and EEG power spectra, considering the role of progesterone’s neuroactive metabolites that interact with the gamma-aminobutyric acid (GABA) receptor complex.
Key findings from the study include:
Increase in Non-REM Sleep: Administration of progesterone significantly increased the amount of non-rapid eye movement (non-REM) sleep in participants.
Changes in EEG Spectral Power: During non-REM sleep, there was a notable decrease in the slow wave frequency range (0.4-4.3 Hz) and a tendency for elevated spectral power in the higher frequency range (>15 Hz). These changes in sleep architecture and EEG power spectra resemble those induced by agonistic modulators of the GABA receptor complex, suggesting that the effects might be mediated through the conversion of progesterone into its GABA-active metabolites, allopregnanolone and pregnanolone.
Interindividual Variability: The study observed significant interindividual variability in the bioavailability of progesterone and its metabolites, which could influence the extent of the effects on sleep EEG.
Potential Mechanism: The effects of progesterone on sleep patterns and EEG power spectra are partially attributed to its conversion into GABA-active metabolites, which modulate the GABA receptor complex, influencing sleep architecture.
Implications for Sleep Disorders: The findings suggest that progesterone and its metabolites could have therapeutic potential in managing sleep disorders, given their influence on sleep architecture and the sleep EEG.
This study contributes to the understanding of how progesterone influences sleep, highlighting the potential role of its neuroactive metabolites in modulating sleep architecture through interactions with the GABA receptor complex.


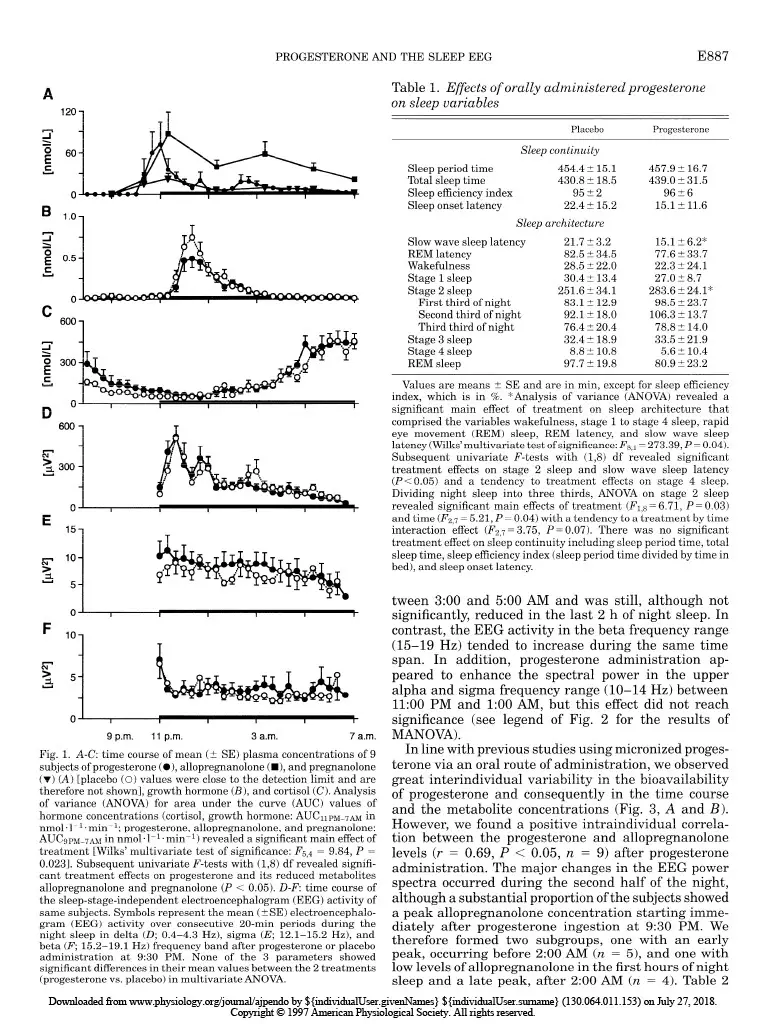
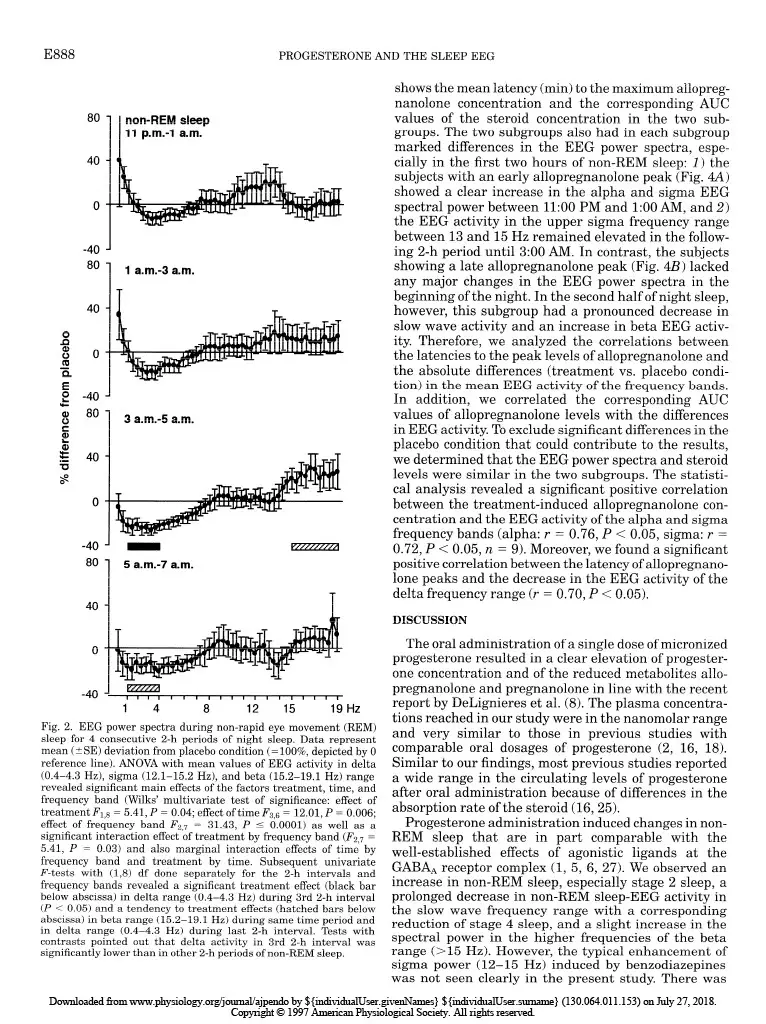
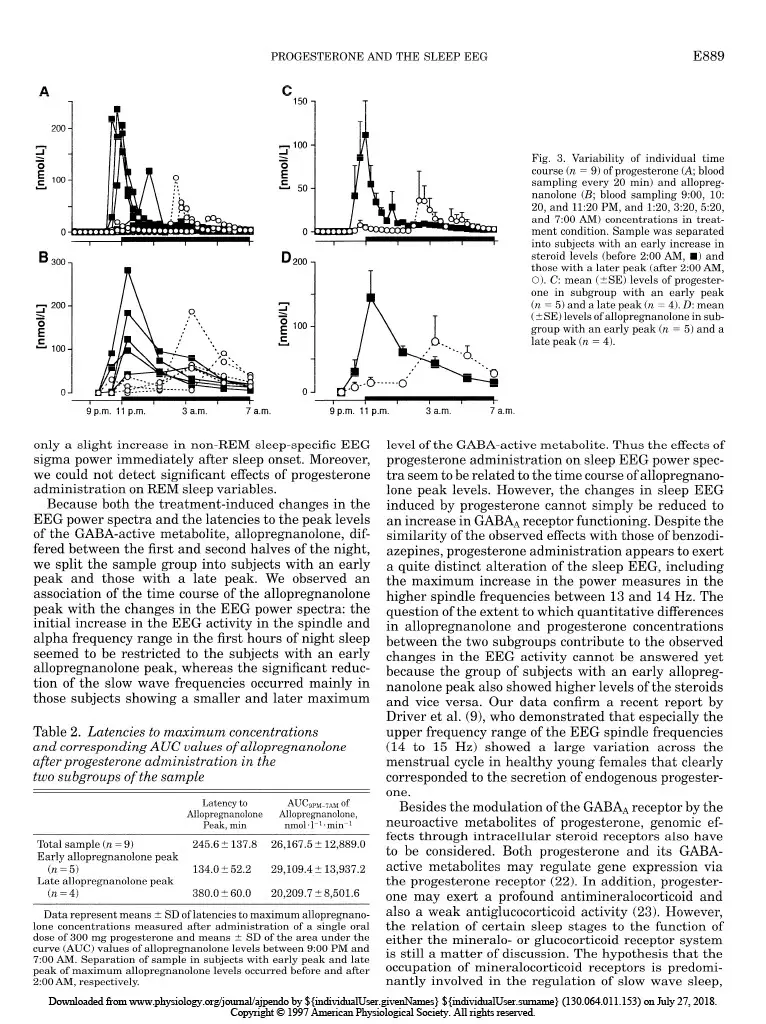
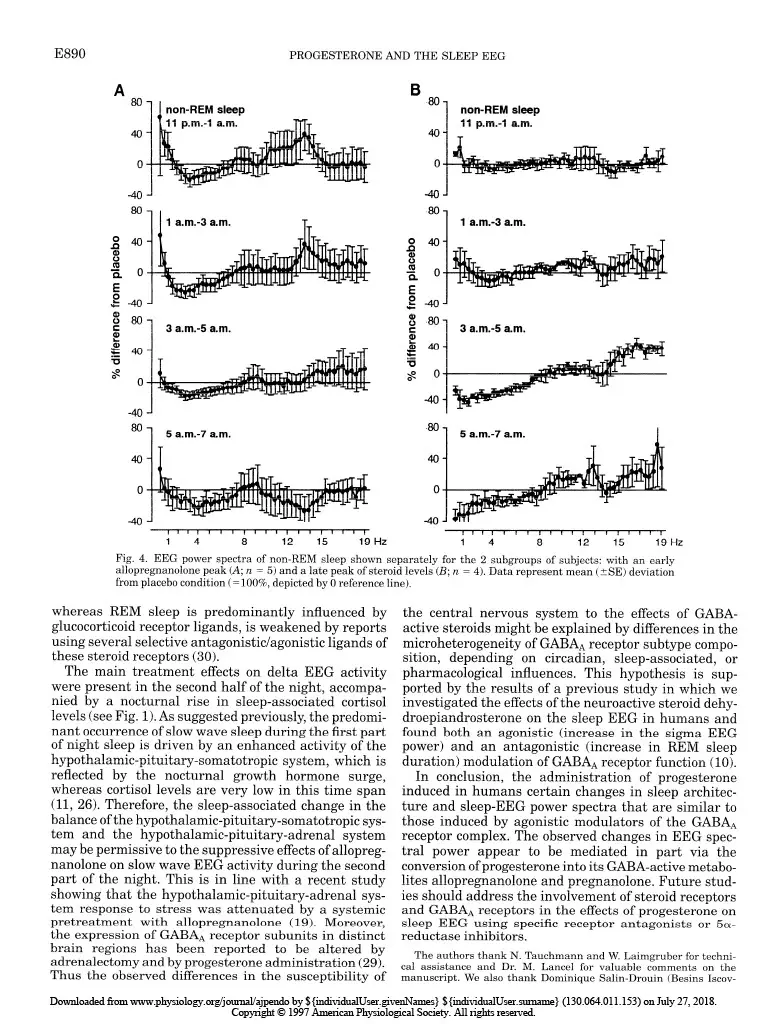

Progesterone-induced changes in sleep in male subjects ELISABETH FRIESS, HIROKUNI TAGAYA, LORENZ TRACHSEL, FLORIAN HOLSBOER, AND RAINER RUPPRECHT Department of Psychiatry, Clinical Institute, Max Planck Institute of Psychiatry, D-80804 Munich, Germany Friess, Elisabeth, Hirokuni Tagaya, Lorenz Trachsel, Florian Holsboer, and Rainer Rupprecht. Progesterone- induced changes in sleep in male subjects. Am. J. Physiol. 272 (EndocrinoZ. Metab. 35): E885-E891, 1997. -Progesterone administration induces a reduction of the vigilance state in humans during wakefulness. It has been been suggested that this effect is mediated via neuroactive metabolites that interact with the y-aminobutyric acid* (GABA*) receptor complex. To investigate the effects of progesterone administra- tion on the sleep electroencephalogram (EEG) in humans we made polysomnographic recordings, including sleep stage- specific spectral analysis, and concomitantly measured plasma concentrations of progesterone and its GABA-active metabo- lites 3a-hydroxy-5a-dihydroprogesterone (allopregnanolone) and 3a-hydroxy-5P-dihydroprogesterone (pregnanolone) in nine healthy male subjects in a double-blind placebo- controlled crossover study. Progesterone administration at 9:30 PM induced a significant increase in the amount of non-rapid eye movement (REM) sleep. The EEG spectral power during non-REM sleep showed a significant decrease in the slow wave frequency range (0.4-4.3 Hz), whereas the spectral power in the higher frequency range (> 15 Hz) tended to be elevated. Some of the observed changes in sleep architec- ture and sleep-EEG power spectra are similar to those induced by agonistic modulators of the GABA* receptor complex and appear to be mediated in part via the conversion of progesterone into its GABA-active metabolites. all-night spectral analysis; electroencephalogram power spec- tra; neurosteroids; allopregnanolone; y-aminobutyric acid* receptor complex A DOSE-DEPENDENT hypnotic effect after intravenous progesterone injections was reported as early as 40 years ago (15). Studies using oral administration of micronized progesterone showed a transient reduction in vigilance during wakefulness (2). Both in vivo and in vitro studies suggest that the central nervous system effects of progesterone are mediated in part through certain metabolites that stereoselectively modulate the y-aminobutyric acid* (GABAA) receptor complex func- tion (4). Especially, the A-ring-reduced metabolites 3a-hydroxy-&dihydroprogesterone (allopregnanolone) and 3a-hydroxy-5P-dihydroprogesterone (pregnano- lone) are potent positive allosteric modulators of the GABA* receptor complex (14). The initial studies of the effects of progestins on the central nervous system excitability in healthy male subjects using quantitative spectral analysis of the electroencephalogram (EEG) during wakefulness indi- cated similarities of the EEG power spectra induced by progestins and benzodiazepines (12). Agonistic ligands of the GABAA receptor complex produce typical effects on sleep architecture: these drugs shorten sleep onset latency, suppress the amount of rapid eye movement (REM) sleep and increase non-REM sleep. Moreover, the sleep-EEG power spectrum induced by benzodiaz- epines shows a characteristic increase in the sigma or spindle frequency activity (12-15 Hz) and a decrease in slow wave activity (l-4 Hz) (1,5,6,27). On the other hand, studies investigating physiologi- cal or pathophysiological states with a distinct pattern of progesterone secretion point to an association of rapid or severe changes in peripheral progesterone concentrations with disturbances of mood and impair- ment of physical well being, vigilance, and sleep qual- ity. Feelings of fatigue during early pregnancy have been attributed to the enhanced levels of progesterone (3). Although high plasma progesterone concentration preceding the clinical symptoms are required for the occurrence of the symptoms during the premenstrual syndrome (PMS), there is evidence that PMS does not reflect simply a deficiency state of progesterone secre- tion (21,24). Spectral analysis of the sleep EEG during the course of pregnancy revealed a progressive reduction of the EEG spectral power, with the largest changes in the spindle frequency range (7). In a recent report of Driver et al. (9) the variations of the spectral power in the upper sigma frequency range have been shown to be related to the menstrual cycle. A sleep study in rats demonstrated a great similarity of the spectral changes induced by progesterone to those of agonistic modula- tors of the GABAA receptor complex (13). However, studies evaluating the effects of progesterone on hu- man sleep EEG are not available to date. Therefore, we performed a sleep-endocrine study in humans using a double-blind, placebo-controlled cross- over design to evaluate the effects of orally adminis- tered progesterone on sleep architecture and sleep- associated hormone secretion. In addition, we used an all-night serial spectral analysis of the sleep EEG to investigate the progesterone-induced effects on slow wave activity and EEG activity in the higher frequen- cies within the sigma and beta range to relate these effects to the pharmacokinetics of progesterone and its GABA-active metabolites allopregnanolone and preg- nanolone. MATERIALS AND METHODS Subjects and study design. To rule out interference from variations in endogenous progesterone secretion we studied only male subjects. The sample group consisted of nine healthy volunteers (mean age t SD: 24.7 t 4.3 yr, range: 19-33 yr) who underwent extensive psychiatric, physical, and laboratory examinations before entering the experiment. Subjects had no sleep disturbances and no family or personal history of psychiatric or neurological disorder, and they had received no medical treatment for the last 3 mo. Shift workers 0193-1849/97 $5.00 Copyright o 1997 the American Physiological Society EBB5 Downloaded from www.physiology.org/journal/ajpendo by ${individualUser.givenNames} ${individualUser.surname} (130.064.011.153) on July 27, 2018. Copyright © 1997 American Physiological Society. All rights reserved.E886 PROGESTERONEANDTHESLEEPEEG and subjects who had recently made a transmeridian flight were excluded. After written informed consent was obtained, the subjects underwent two experimental sessions separated by at least 1 wk. Each experimental session consisted of two consecutive nights in our sleep laboratory; the first night served for adaptation to the laboratory setting. On the evening of the following night the subjects received an electrolyte-balanced meal at 7:00 PM. An indwelling forearm catheter was inserted at 7:30 PM. At 9:30 PM a single oral dose of 300 mg micronized progesterone (Utrogestan, Besins Iscovesco Laboratoires, Paris, France) or placebo was admin- istered according to a double-blind randomized schedule. Blood sampling started at 800 PM from an adjacent room by means of a long plastic tube that was connected to the forearm catheter. Blood specimens were collected every 20 min through- out the whole observation period without disturbing the subjects’ sleep. Until “lights off’ (II:00 PM) the subjects remained in a semirecumbent position. They were allowed to watch TV, read, or listen to music. The experimental protocol was approved by the Ethics Committeee for Human Experiments of the Max Planck Institute of Psychiatry. Sleep variables and EEGpower spectra. Polysomnographic sleep recordings [2 EEGs: C3-A2/C4-Al, high-pass filter at 0.5 Hz, notch filter at 50 Hz, vertical and horizontal electroocu- logram (EOG), electromyogram (EMG), and electrocardio- gram (ECG)] were obtained from 1l:OO PM to 7:00 AM. Sleep stages were visually scored according to standard guidelines (20) by experienced raters who were not aware of the treat- ment condition. The EOG, EEG, EMG, and ECG signals were filtered and transmitted to the polygraph (Schwartzer, ED 24). The digitalized data (8 bit analog-to-digital converter, sampling rate 100 Hz) were stored on disk and calibrated with a 50-PV IO-Hz sine wave. The C3-A2/C4-Al EEG derivations were submitted to a spectral analysis using a fast-Fourier transformation. The EEG power spectra were computed as described previously (28). The frequency resolu- tion was 0.39 Hz between inclusive 0 and 48.3 Hz. To exclude possible aliasing due to the filter settings, the analysis was restricted to 0 and 19.1 Hz. State-specific power of 50 frequency bins at 0.39-Hz intervals was cumulated across the delta (0.4-4.3 Hz), theta (4.3-7.8 Hz), alpha (7.8-12.1 Hz), sigma (12.1-15.2 Hz), and beta (15.2-19.1 Hz) range. Hormone analysis. Plasma cortisol (ICN Biomedicals, Car- son, CA), growth hormone (Nichols Institute, San Juan Capistrano, CA), and progesterone (ICN Biomedicals) concen- trations were determined from all samples by commercially available radioimmunoassay kits. The detection limit for cortisol was 0.83 nmol/l, for growth hormone 0.09 nmol/l, and for progesterone 0.06 nmol/l. The intra- and interassay coefficients of variation for cortisol, growth hormone, and progesterone determinations were all ~7%. All samples of one subject were measured in duplicate in one assay. The samples obtained at 9:00,10:20, and 11:20 PM and 1:20,3:20, 5:20, and 7:00 AM were further quantified for the concentra- tions of the metabolites allopregnanolone and pregnanolone. The measurements were performed with combined gas chro- matography-mass spectrometry using an HP 5988 mass spectrometer focused to monitor the negative ions generated in the chemical ionization mode with methane as the reactant gas (CEMAF, Poitiers, France). The compounds and Sp- pregnanolone, added as the internal standard, were isolated from plasma by solid-liquid extraction. The recovery rate was 70%, and the lower detection limit was 1.6 nmol/I. The relative standard deviation at these concentrations was 7.1% for allopregnanolone and 1.9% for pregnanolone. Statistical analyses. Treatment effects on EEG power spec- tra, polysomnographic, and hormonal data were tested about significance by multivariate analysis of variance (MANOVA) with repeated-measures design. Thereby, treatment was a within-subjects factor with two levels (progesterone vs. pla- cebo). In case of significant treatment effects, the identifica- tion of the variables that contributed to this effect was carried out by subsequent univariate F-tests. Whenever time was also considered as a within-subjects factor, differences be- tween the various time levels were tested for significance by tests with contrasts. For the hormonal variables the area under the curve (AUC) was used in the statistical analysis. As nominal level of significance a = 0.05 was accepted. To keep the type I error (0.05 all posteriori tests were performed at a reduced level of significance (Bonferroni adjusted alpha). For quantifying and testing associations between some variables the Pearson’s product-moment correlation coefficient was used. RESULTS Hormone measurements. As shown in Fig. 1, adminis- tration of progesterone resulted in a ma rked in crease in progesterone and the reduced metabolites allopregnano- lone and pregnanolone (see legend of Fig. 1 for the results of the MANOVA), respectively. In the placebo condition, plasma concentrations of progesterone, allo- pregnanolone, and pregnanolone were consistently close to the detection limits and are therefore not shown in the graph. The secretion of the sleep-associated hor- mones cortisol (Fig. 1B) and growth hormone (Fig. 1C) did not differ significantly between treatment and placebo conditions. Sleep variables. The results obtained by conventional scoring of the sleep stages according to the standard criteria are listed in Table 1. Progesterone administra- tion induced a significant change in the sleep architec- ture, namely an increase in the time spent in stage 2 sleep compared with placebo. This effect occurred pre- dominantly in the first one-third of the night. In contrast, there was a tendency toward a decrease in stage 4 sleep and also a reduction of REM sleep, which did not reach statistical significance. The slow wave sleep latency (referring to 1st stage 2 sleep) was significantly shortened. None of the other sleep vari- ables, including measures for sleep continuity, were significantly altered by progesterone administration (see legend of Table 1 for the results of the MANOVA). EEG power spectra. The time courses of the EEG activity over all sleep stages in the delta, sigma, and beta frequency range are shown in Fig. 1, D-F. There was a slight reduction of the delta EEG activity and an increase in the beta EEG activity around 3:00 AM after progesterone administration, whereas the sigma EEG activity seemed to be enhanced in the first hours of the night. However, their mean values calculated over the whole night did not show significant differences be- tween the progesterone and placebo conditions. For further investigations of the treatment and time effects on the EEG power densities in these frequency bands, we computed the sleep stage-specific EEG power spec- tra of non-REM sleep during four consecutive 2-h intervals of night sleep. As demonstrated in Fig. 2, the spectral power in the delta frequency range (l-4 Hz) declined progressively to a significant decrease be- Downloaded from www.physiology.org/journal/ajpendo by ${individualUser.givenNames} ${individualUser.surname} (130.064.011.153) on July 27, 2018. Copyright © 1997 American Physiological Society. All rights reserved.PROGESTERONE AND THE SLEEP EEG EBB7 A 2 o 60 E c C 600 1 n 0 Y 600 1 g 10 .=i: 5 1 0 s I b I I I I- 0 ‘ I I I I I t 9 p.m. 11 p.m. 3 a.m. 7 a.m. Fig. 1. A-C: time course of mean (t SE) plasma concentrations of 9 subjects of progesterone (a), allopregnanolone ( n ), and pregnanolone (v) (A) [placebo (0) values were close to the detection limit and are therefore not shown], growth hormone (B), and cortisol (C). Analysis of variance (ANOVA) for area under the curve (AUC) values of hormone concentrations (cortisol, growth hormone: AUC~PM-~~ in nmol * 1-l * min-l; progesterone, allopregnanolone, and pregnanolone: AUC~PM-~~ in nmol -1-l. min-l) revealed a significant main effect of treatment [Wilks’ multivariate test of significance: F5,4 = 9.84, P = 0.0231. Subsequent univariate F-tests with (1,8) df revealed signifi- cant treatment effects on progesterone and its reduced metabolites allopregnanolone and pregnanolone (P < 0.05). D-F: time course of the sleep-stage-independent electroencephalogram (EEG) activity of same subjects. Symbols represent the mean (+ SE) electroencephalo- gram (EEG) activity over consecutive 20-min periods during the night sleep in delta (II; 0.4-4.3 Hz), sigma (E; 12.1-X.2 Hz), and beta (F; X2-19.1 Hz) frequency band after progesterone or placebo administration at 9:30 PM. None of the 3 parameters showed significant differences in their mean values between the 2 treatments (progesterone vs. placebo) in multivariate ANOVA. Table 1. Effects of orally administered progesterone on sleep variables Placebo Progesterone Sleep continuity Sleep period time 454.4 iI 15.1 457.9 + 16.7 Total sleep time 430.8 2 18.5 439.0 L 31.5 Sleep efficiency index 9522 9626 Sleep onset latency 22.4 t 15.2 15.15 11.6 Sleep architecture Slow wave sleep latency 21.7 + 3.2 15.12 6.2* REM latency 82.5 + 34.5 77.6 + 33.7 Wakefulness 28.5 2 22.0 22.3 L 24.1 Stage 1 sleep 30.4 t 13.4 27.0 + 8.7 Stage 2 sleep 251.6 + 34.1 283.6 + 24.1* First third of night 83.17t 12.9 98.5 + 23.7 Second third of night 92.12 18.0 106.3 + 13.7 Third third of night 76.4 5 20.4 78.8 + 14.0 Stage 3 sleep 32.4 t 18.9 33.5 + 21.9 Stage 4 sleep 8.8 Ilt 10.8 5.6 + 10.4 REM sleep 97.7 5 19.8 80.9 t 23.2 Values are means + SE and are in min, except for sleep efficiency index, which is in %. *Analysis of variance (ANOVA) revealed a significant main effect of treatment on sleep architecture that comprised the variables wakefulness, stage 1 to stage 4 sleep, rapid eye movement (REM) sleep, REM latency, and slow wave sleep latency (Wilks’multivariate test of significance: F~,J = 273.39, P = 0.04). Subsequent univariate F-tests with (1,8) df revealed significant treatment effects on stage 2 sleep and slow wave sleep latency (PC 0.05) and a tendency to treatment effects on stage 4 sleep. Dividing night sleep into three thirds, ANOVA on stage 2 sleep revealed significant main effects of treatment (F1,8 = 6.71, P = 0.03) and time (F 2,7 = 5.21, P = 0.04) with a tendency to a treatment by time interaction effect (F 2,7 = 3.75, P= 0.07). There was no significant treatment effect on sleep continuity including sleep period time, total sleep time, sleep eficiency index (sleep period time divided by time in bed), and sleep onset latency. tween 3:00 and 500 AM and was still, although not significantly, reduced in the last 2 h of night sleep. In contrast, the EEG activity in the beta frequency range (15-19 Hz) tended to increase during the same time span. In addition, progesterone administration ap- peared to enhance the spectral power in the upper alpha and sigma frequency range (lo-14 Hz) between 11:OO PM and 1:00 AM, but this effect did not reach significance (see legend of Fig. 2 for the results of MANOVA). In line with previous studies using micronized proges- terone via an oral route of administration, we observed great interindividual variability in the bioavailability of progesterone and consequently in the time course and the metabolite concentrations (Fig. 3, A and B). However, we found a positive intraindividual correla- tion between the progesterone and allopregnanolone levels (r = 0.69, P < 0.05, n = 9) after progesterone administration. The major changes in the EEG power spectra occurred during the second half of the night, although a substantial proportion of the subjects showed a peak allopregnanolone concentration starting imme- diately after progesterone ingestion at 9:30 PM. We therefore formed two subgroups, one with an early peak, occurring before 2:00 AM (n = 5), and one with low levels of allopregnanolone in the first hours of night sleep and a late peak, after 200 AM (n = 4). Table 2 Downloaded from www.physiology.org/journal/ajpendo by ${individualUser.givenNames} ${individualUser.surname} (130.064.011.153) on July 27, 2018. Copyright © 1997 American Physiological Society. All rights reserved.E888 PROGESTERONE AND THE SLEEP EEG 80 non-REM deep 11 p.m.4 a.m. -40 ‘ 80 1 a.m.-3 a.m. 0 a i!i 0 s F 0 t -40 8 80 1 3 a.m.4 a.m. ii -40 ‘ B 80 1 5 a.m.-7 a.m. 0 Fig. 2. EEG power spectra during non-rapid eye movement (REM) sleep for 4 consecutive 2-h periods of night sleep. Data represent mean (?SE) deviation from placebo condition (= lOO%, depicted by 0 reference line). ANOVA with mean values of EEG activity in delta (0.4-4.3 Hz), sigma (12.1-15.2 Hz), and beta (15.2-19.1 Hz) range revealed significant main effects of the factors treatment, time, and frequency band (Wilks’ multivariate test of significance: effect of treatment F 1,8 = 5.41, P = 0.04; effect of time I$ = 12.01, P = 0.006; effect of frequency band &,7 = 31.43, P 5 0.0001) as well as a significant interaction effect of treatment by frequency band (I$,7 = 5.41, P = 0.03) and also marginal interaction effects of time by frequency band and treatment by time. Subsequent univariate F-tests with (1,8) df done separately for the 2-h intervals and frequency bands revealed a significant treatment effect (black bar below abscissa) in delta range (0.4-4.3 Hz) during 3rd 2-h interval (P < 0.05) and a tendency to treatment effects (hatched bars below abscissa) in beta range (15.2-19.1 Hz) during same time period and in delta range (0.4-4.3 Hz) during last 2-h interval. Tests with contrasts pointed out that delta activity in 3rd 2-h interval was significantly lower than in other 2-h periods of non-REM sleep. shows the mean latency (min) to the maximum ailopreg- nanolone concentration and the corresponding AUC values of the steroid concentration in the two sub- groups. The two subgroups also had in each subgroup marked differences in the EEG power spectra, espe- cially in the first two hours of non-REM sleep: 1) the subjects with an early allopregnanolone peak (Fig. 4A) showed a clear increase in the alpha and sigma EEG spectral power between 11:00 PM and 1:00 AM, and 2) the EEG activity in the upper sigma frequency range between 13 and 15 Hz remained elevated in the follow- ing Z-h period until 3:00 AM. In contrast, the subjects showing a late allopregnanolone peak (Fig. 4B) lacked any major changes in the EEG power spectra in the beginning of the night. In the second half of night sleep, however, this subgroup had a pronounced decrease in slow wave activity and an increase in beta EEG activ- ity. Therefore, we analyzed the correlations between the latencies to the peak levels of allopregnanolone and the absolute differences (treatment vs. placebo condi- tion) in the mean EEG activity of the frequency bands. In addition, we correlated the corresponding AUC values of allopregnanolone levels with the differences in EEG activity. To exclude significant differences in the placebo condition that could contribute to the results, we determined that the EEG power spectra and steroid levels were similar in the two subgroups. The statisti- cal analysis revealed a significant positive correlation between the treatment-induced allopregnanolone con- centration and the EEG activity of the alpha and sigma frequency bands (alpha: r = 0.76, P < 0.05, sigma: r = 0.72, P < 0.05, n = 9). Moreover, we found a significant positive correlation between the latency of allopregnano- lone peaks and the decrease in the EEG activity of the delta frequency range (r = 0.70, P < 0.05). DISCUSSION The oral administration of a single dose of micronized progesterone resulted in a clear elevation of progester- one concentration and of the reduced metabolites allo- pregnanolone and pregnanolone in line with the recent report by DeLignieres et al. (8). The plasma concentra- tions reached in our study were in the nanomolar range and very similar to those in previous studies with comparable oral dosages of progesterone (2, 16, 18). Similar to our findings, most previous studies reported a wide range in the circulating levels of progesterone after oral administration because of differences in the absorption rate of the steroid (16,25). Progesterone administration induced changes in non- REM sleep that are in part comparable with the well-established effects of agonistic ligands at the GABA* receptor complex (1, 5, 6, 27). We observed an increase in non-REM sleep, especially stage 2 sleep, a prolonged decrease in non-REM sleep-EEG activity in the slow wave frequency range with a corresponding reduction of stage 4 sleep, and a slight increase in the spectral power in the higher frequencies of the beta range (X5 Hz). However, the typical enhancement of sigma power (12-15 Hz) induced by benzodiazepines was not seen clearly in the present study. There was Downloaded from www.physiology.org/journal/ajpendo by ${individualUser.givenNames} ${individualUser.surname} (130.064.011.153) on July 27, 2018. Copyright © 1997 American Physiological Society. All rights reserved.PROGESTERONE AND THE SLEEP EEG EBB9 C B 300- 3 200- A E = loo- O- 150 D 200 0 Fig. 3. Variability of individual time course (n = 9) of progesterone (A; blood sampling every 20 min) and allopreg- nanolone (I?; blood sampling 9:00, 10: 20, and 11:20 PM, and 1:20, 3:20, 520, and 7:00 AM) concentrations in treat- ment condition. Sample was separated into subjects with an early increase in steroid levels (before 2:00 AM, H) and those with a later peak (after 2:00 AM, 0). C: mean (&SE) levels of progester- one in subgroup with an early peak (n = 5) and a late peak (n = 4). D: mean (+ SE) levels of allopregnanolone in sub- group with an early peak (n = 5) and a R T 9 p.m. 11 p.m. 3 a.m. 7 a.m. 9 p.m. 11 p.m. 3 a.m. 7 a.m. only a slight increase in non-REM sleep-specific EEG sigma power immediately after sleep onset. Moreover, we could not detect significant effects of progesterone administration on REM sleep variables. Because both the treatment-induced changes in the EEG power spectra and the latencies to the peak levels of the GABA-active metabolite, allopregnanolone, dif- fered between the first and second halves of the night, we split the sample group into subjects with an early peak and those with a late peak. We observed an association of the time course of the allopregnanolone peak with the changes in the EEG power spectra: the initial increase in the EEG activity in the spindle and alpha frequency range in the first hours of night sleep seemed to be restricted to the subjects with an early allopregnanolone peak, whereas the significant reduc- tion of the slow wave frequencies occurred mainly in those subjects showing a smaller and later maximum Table 2. Latencies to maximum concentrations and corresponding AUC values of allopregnanolone after progesterone administration in the two subgroups of the sample Latency to AUCSPM-‘7AM of Allopregnanolone Allopregnanolone, Peak, min nmol-l-l-min-l Total sample (n = 9) 245.6 + 137.8 26J67.5 5 12,889.0 Early allopregnanolone peak (n = 5) 134.0 t 52.2 29JO9.4 + 13,937.2 Late allopregnanolone peak (n = 4) 380.0 + 60.0 20,209.7 2 8,501.6 Data represent means + SD of latencies to maximum allopregnano- lone concentrations measured after administration of a single oral dose of 300 mg progesterone and means ? SD of the area under the curve (AUC) values of allopregnanolone levels between 9:00 PM and 7:00 AM. Separation of sample in subjects with early peak and late peak of maximum allopregnanolone levels occurred before and after 2:00 AM, respectively. level of the GABA-active metabolite. Thus the effects of progesterone administration on sleep EEG power spec- tra seem to be related to the time course of allopregnano- lone peak levels. However, the changes in sleep EEG induced by progesterone cannot simply be reduced to an increase in GABA* receptor functioning. Despite the similarity of the observed effects with those of benzodi- azepines, progesterone administration appears to exert a quite distinct alteration of the sleep EEG, including the maximum increase in the power measures in the higher spindle frequencies between 13 and 14 Hz. The question of the extent to which quantitative differences in allopregnanolone and progesterone concentrations between the two subgroups contribute to the observed changes in the EEG activity cannot be answered yet because the group of subjects with an early allopreg- nanolone peak also showed higher levels of the steroids and vice versa. Our data confirm a recent report by Driver et al. (9), who demonstrated that especially the upper frequency range of the EEG spindle frequencies (14 to 15 Hz) showed a large variation across the menstrual cycle in healthy young females that clearly corresponded to the secretion of endogenous progester- one. Besides the modulation of the GABAA receptor by the neuroactive metabolites of progesterone, genomic ef- fects through intracellular steroid receptors also have to be considered. Both progesterone and its GABA- active metabolites may regulate gene expression via the progesterone receptor (22). In addition, progester- one may exert a profound antimineralocorticoid and also a weak antiglucocorticoid activity (23). However, the relation of certain sleep stages to the function of either the mineralo- or glucocorticoid receptor system is still a matter of discussion. The hypothesis that the occupation of mineralocorticoid receptors is predomi- nantly involved in the regulation of slow wave sleep, Downloaded from www.physiology.org/journal/ajpendo by ${individualUser.givenNames} ${individualUser.surname} (130.064.011.153) on July 27, 2018. Copyright © 1997 American Physiological Society. All rights reserved.E890 PROGESTERONE AND THE SLEEP EEG -40 1 non-REM sleep I 1 a.m.4 a.m. T T 3 a.m.-5 a.m. 80 1 5 a.m.-7 a.m. – Ii -T B e80 1 a.m.03 a.m. 3 a.m.95 a.m. 430 1 5 a.m.-7 a.m. I 1 4 8 12 15 19Hz 1 4 8 12 15 19 Hz Fig. 4. EEG power spectra of non-REM sleep shown separately for the 2 subgroups of subjects: with an early allopregnanolone peak (A; IZ = 5) and a late peak of steroid levels (B; n = 4). Data represent mean (&SE) deviation from placebo condition (= lOO%, depicted by 0 reference line). whereas REM sleep is predominantly influenced by glucocorticoid receptor ligands, is weakened by reports using several selective antagonistic/agonistic ligands of these steroid receptors (30). The main treatment effects on delta EEG activity were present in the second half of the night, accompa- nied by a nocturnal rise in sleep-associated cortisol levels (see Fig. 1). As suggested previously, the predomi- nant occurrence of slow wave sleep during the first part of night sleep is driven by an enhanced activity of the hypothalamic-pituitary-somatotropic system, which is reflected by the nocturnal growth hormone surge, whereas cortisol levels are very low in this time span (11, 26). Therefore, the sleep-associated change in the balance of the hypothalamic-pituitary-somatotropic sys- tem and the hypothalamic-pituitary-adrenal system may be permissive to the suppressive effects of allopreg- nanolone on slow wave EEG activity during the second part of the night. This is in line with a recent study showing that the hypothalamic-pituitary-adrenal sys- tem response to stress was attenuated by a systemic pretreatment with allopregnanolone (19). Moreover, the expression of GABAA receptor subunits in distinct the central nervous system to the effects of GABA- active steroids might be explained by differences in the microheterogeneity of GABAA receptor subtype compo- sition, depending on circadian, sleep-associated, or pharmacological influences. This hypothesis is sup- ported by the results of a previous study in which we investigated the effects of the neuroactive steroid dehy- droepiandrosterone on the sleep EEG in humans and found both an agonistic (increase in the sigma EEG power) and an antagonistic (increase in REM sleep duration) modulation of GABAA receptor function (10). In conclusion, the administration of progesterone induced in humans certain changes in sleep architec- ture and sleep-EEG power spectra that are similar to those induced by agonistic modulators of the GABA* receptor complex. The observed changes in EEG spec- tral-power appear to be mediated in part via the conversion of progesterone into its GABA-active metabo- lites allopregnanolone and pregnanolone. Future stud- ies should address the involvement of steroid receptors and GABAA receptors in the effects of progesterone on sleep EEG using specific receptor antagonists or 5~ reductase inhibitors. brain regions has been reported to be altered by adrenalectomy and by progesterone administration (29). The authors thank N. Tauchmann and W. Laimgruber for techni- Thus the observed differences in the susceptibility of cal assistance and Dr. M. Lance1 for valuable comments on the manuscript. We also thank Dominique Salin-Drouin (Besins Iscov- Downloaded from www.physiology.org/journal/ajpendo by ${individualUser.givenNames} ${individualUser.surname} (130.064.011.153) on July 27, 2018. Copyright © 1997 American Physiological Society. All rights reserved.PROGESTERONE AND THE SLEEP EEG E891 esco Laboratoires, Paris, France) for providing micronized progester- 15. one (Utrogestan) and for valuable help and discussions. Moreover, we gratefully acknowledge the help of CEMAF (Portiers, France) and Biotec (Orleans, France) with the steroid analysis. 16. This work was supported by the Gerhard-Hess-Program of the Deutsche Forschungsgemeinschaft to R. Rupprecht and by Besins Iscovesco Laboratoires. Address for reprint requests: E. Friess, Max Planck Institute of 17. Psychiatry, Clinical Institute, Dept. of Psychiatry, Kraepelinstr. 10, D-80804 Munich, Germany. 18. Received 26 September 1996; accepted in final form 6 January 1997.
